

Vaccination is one of the most important measures for disease prevention. In this sense, Butantan contributes to the Brazilian public health with the production of several vaccines against viruses and bacteria, generating products of high quality, safety and efficacy.
There are seven types of vaccines supplied to the Ministry of Health, whose current production capacity is the result of in-house development of processes to obtain vaccine antigens, in addition to technology transfer processes and PDPs (Partnerships for Productive Development) between Butantan and external laboratories.
Butantan’s Influenza vaccine protects against three types of Influenza virus. Its composition changes annually due to the high mutation rate of the virus. It is produced by virus inoculation into embryonated chicken eggs. After an incubation period, the allantoic fluid that surrounds the embryo is collected, centrifuged, concentrated, fragmented and inactivated, resulting in a suspension of the monovalent vaccine. Mixing the suspensions of each monovalent thus results in the trivalent vaccine.
The adsorbed hepatitis A vaccine (inactivated) is used to prevent infection caused by the hep A virus. It is produced from a hep A virus, propagated in human cell culture, then purified and inactivated.
The adsorbed hepatitis B vaccine (recombinant) is used to prevent infection caused by the hep B virus, being produced from recombinant yeast Pichia angusta (H.polymorpha) that express the surface antigen of the hep B virus. The Butantan vaccine is the only recombinant vaccine for use fully developed and produced in Brazil.
The human papillomavirus vaccine types 6, 11, 16 and 18 (recombinant) is used to prevent infection caused by the human papillomavirus (HPV), responsible for the occurrence of genital cancer in both women and men. The viral proteins of HPV types 6, 11, 16 and 18 are produced separately in Saccharomyces cerevisiae yeasts, to be formulated in a single quadrivalent dose.
The rabies vaccine is used to prevent human rabies when there is a need to protect individuals exposed to the virus as a result of contact with the saliva of transmitting animals or as prevention in people with occupational exposure to the risk of virus infection. The Butantan vaccine is produced in a cell line (VERO cells), without the use of components of animal origin, and has a high degree of purity and an efficient immune response.
For the production of the triple bacterial vaccine (DTP), the whole cell vaccine of
Bordetella pertussis, the agent that causes pertussis, is manufactured
separately, combined with tetanus and diphtheria toxoids, resulting from the
fermentation of Corynebacterium diphtheriae and Clostridium tetani bacteria to
obtain the toxins and subsequent detoxification.
The composition of tetanus toxoid with diphtheria toxoid generates to the dual
adult (dT) vaccine and the dual infant (DT) vaccine.
Recommended for children under seven years of age who have had adverse events that contraindicate another dose of whole-cell DTP vaccine, and in pregnant women as a booster or complementation of the dual adult vaccine regimen (diphtheria and tetanus), to reduce the mortality of whooping cough in newborns. This vaccine is made up of highly purified parts of Bordetella pertussis combined with diphtheria and tetanus toxoids.
A live attenuated vaccine developed in partnership with Valneva for the prevention of chikungunya, which is classified by the World Health Organization (WHO) as a Neglected Tropical Disease. The vaccine has been approved for use in Brazil, Canada, and Europe.
This vaccine results from a technology transfer agreement with Pfizer and aims to prevent mild and severe lower respiratory tract disease caused by RSV in infants up to six months of age through maternal immunization, as well as in individuals aged 60 years and older.
The dengue vaccine developed by Instituto Butantan, composed of the four attenuated virus serotypes, is the first single-dose vaccine against the disease in the world. It was approved by the Brazilian regulatory agency in November 2025, following the conclusion of the phase 3 clinical trial.

For over a century, Butantan has produced different types of serum against
toxins from venomous animals and microorganisms. Its production involves
immunizing horses with antigens produced from venoms, toxins or viruses,
obtaining different types of plasma, which are subjected to industrial purification
and formulation, resulting in products of high quality, safety and efficacy.
Butantan antitoxins and antivenoms are specific immunoglobulin fractions
purified, presented in liquid form, in vials of 5, 10 or 20 milliliters (depending on
the type) and which must be kept in a refrigerator until use.
Indicated in the treatment of envenomation by snakes of the Bothrops genus (pit vipers). Doses vary according to severity
Indicated in the treatment of envenomation by snakes of the Bothrops genus (pit vipers), or in accidents by Lachesis (bushmaster), found mainly in the Amazon.
Indicated in the treatment of envenomation by Crotalus durissus (South American rattlesnakes). Doses vary according to severity.
Indicated in the treatment of envenomation by snakes of the Bothrops genus (pit vipers) or in accidents by Crotalus durissus (South American rattlesnakes).
Indicated in the treatment of envenomation by snakes of the Micrurus genus (true coral snake).
Indicated in the treatment of envenomation by scorpions of the Tityus genus (yellow, brown or black scorpions), when recommended.
Indicated in the treatment of envenomation by scorpions of the Tityus genus (yellow, brown or black scorpions), or spiders of the Phoneutria genus (Brazilian wandering/armed spider) or Loxosceles (brown spider).
Indicated in the treatment of envenomation by caterpillars of the Lonomia genus (taturana, oruga, tapuru), when recommended.
Indicated to neutralize the toxin of the bacterium Clostridium diphtheriae, present in the bloodstream, according to the type and severity of the disease.
Indicated for the prevention or treatment of accidental or neonatal tetanus, depending on the type and conditions of the wound, as well as the condition of previous tetanus vaccination.
Indicated to neutralize the toxin of the Clostridium botulinum bacterium, which causes botulism.
Indicated for the prophylaxis of human rabies after exposure to the rabies virus, depending on the nature of the exposure and the conditions of the rabid animal attack.
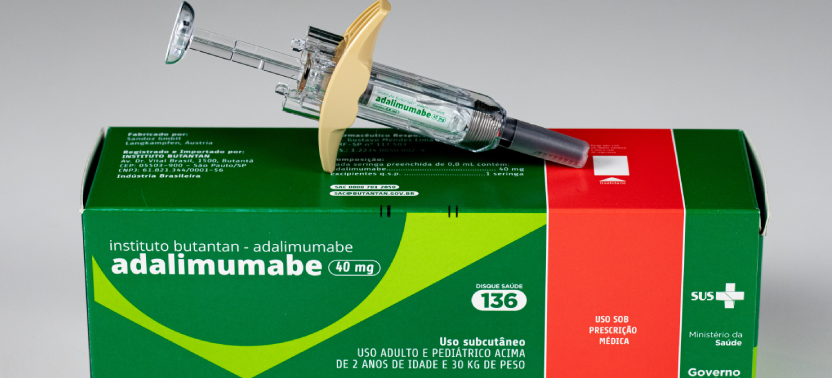
Monoclonal antibodies are advanced therapies indicated for different autoimmune conditions, chronic inflammatory diseases, and cancer.
The main advantages of monoclonal antibodies are their speed and precision in combating diseases.
Since May 2025, Butantan supplies the Ministry of Health with Adalimumab, produced through technology transfer.
This medication is approved for adult and pediatric use in patients over two years of age and weighing at least 30 kg. It is indicated for the treatment of rheumatoid arthritis, psoriatic arthritis, Crohn’s disease, psoriasis, non-infectious uveitis, among other conditions. Its use in Brazil is approved by Anvisa.